Drug-related hypersensitivity due to Abacavir induced ADR
In the previous article Drug related hypersensitivity, prevalence, diagnosis and therapeutic considerations, we discussed about how different drugs lead to different allergic responses. However, it is important that mechanism of different drug is studied which leads to ADR. In the present article Abacavir, a drug which is used for the treatment and prevention of Human Immuno Deficiency Virus (HIV) (Hewitt et al., 2002) is being discussed. Abacavir is a synthetic carbocyclic nucleoside which is categorised under the class of Nucleoside Reverse Transcriptase Inhibitors.
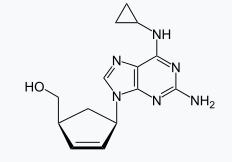
The molecular weight of the drug causes hypersensitivity reaction which activates the immune response faster by covalently binding to the self-protein producing a haptenated molecule which is then presented to the adaptive immune system to induce an immune response.
Mode of Action in HIV infected Patients
Abacavir acts by incorporating itself into the DNA of the virus which stops the building process of transcription from RNA to DNA. The resulting DNA is incomplete which causes reverse transcriptase process thus halting the virus development process. The carbovir triphosphate lacks a 3’ –OH group and thus blocks the HIV replication and blocks the HIV formation and stops the formation of new cells (De Clercq, 2009) (See Figure 2 below).

It is rapidly absorbed in the form of oral dosage. Maximum concentration of the drug reached in about 0.8 hours after dosing. About 98% of drug goes under extensive hepatic transformation via the alcohol dehydrogenase and glucuronyl transferase enzymes to inactive metabolites. About 50% of the drug Abacavir bounds to the plasma proteins. Symptoms of the hypersensitivity reaction caused by the abacavir are fever, rash on skin, gastrointestinal including nausea, vomiting, diarrhea, abdominal pain, constitutional including generalized malaise, fatigue and ache, changes in the body fat, respiratory including cough, pharyngitis. Abacavir commonly causes anaphylaxis, liver failure. Sometimes it causes mouth ulceration, lymphadenopathy.
Hypersensitivity due to Abacavir allergy
Furthermore, the world statistics, although Abacavir is a well tolerated drug, however hypersensitivity due to the drug is found among 5-8% of the total world population mostly mediated by activation of HLA-B*5701 due to MHC-I presentation of derivative Abacavir leading to disruption of normal activity (Chessman et al 2008, Zhang, et al, 2012). This ultimately leads to hypersensitivity and is detected within 6 weeks of treatment through symptoms like fever, rashes, GI tract sensitivity, and respiratory problems (Hetherington, et al, 2001). Among the case countries, maximum incidence is found in European population i.e. 5%, followed by 1% prevalence in Asian population and less than 1% in African population. Among treatment options, low fat diet and aerobic exercises are suggested. Further, drugs like, Thiazolidiones and Metformin have been found to improve immune response to sensitivity and Gemfibrozil and atorvastatin helps in lowering the lipids (Marfatia & Makrandi Smita, 2005).
Reference
- Barbarino Julia M, Kroetz Deanna L, Altman Russ B, Klein Teri E . “PharmGKB summary: abacavir pathway”Pharmacogenetics and genomics (2014). https://www.pharmgkb.org/pmid/24625462.
- Banerjee, M., & Thomas, R. (2005). HLA-A allele frequency and haplotype distribution in the dravidian tribal communities of south India. Indian Journal of Human Genetics, 11(3), 140. https://doi.org/10.4103/0971-6866.19533.
- Baniasadi, S., Shokouhi, S. B., Tabarsi, P., Alehashem, M., Khalili, H., Fahimi, F., & Nadji, S. A. (2016). Prevalence of HLA-B*5701 and Its Relationship with Abacavir Hypersensitivity Reaction in Iranian HIV-Infected Patients. Tanaffos, 15(1), 48–52. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27403179.
- Chakravarty, J., Sharma, S., Johri, A., Chourasia, A., & Sundar, S. (2016). Clinical Abacavir Hypersensitivity Reaction among Children in India. The Indian Journal of Pediatrics, 83(8), 855–858. https://doi.org/10.1007/s12098-016-2044-z.
- Chessman D, Koslenko L, Lethborg T., Purcell AW, Williamson NA, Chen Z. Human Leukocyte antigen class-I restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008 Jun 28(6): 822-32.
- Coopman, S. A., Johnson, R. A., & Platt, R. (2016). Cutaneous Disease and Drug Reactions in HIV Infections. The New England Journal of Medicine Downloaded from Nejm.org on, 2–4. Retrieved from www.nejm.org/doi/pdf/10.1056/NEJM199306103282304.
- De Clercq, E. (2009). Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV! International Journal of Antimicrobial Agents, 33, 307–320. https://doi.org/10.1016/j.ijantimicag.2008.10.010.
- Gonzalez-Galarza, F. F., Christmas, S., Middleton, D., & Jones, A. R. (2011). Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Research, 39(Database issue), D913-9. https://doi.org/10.1093/nar/gkq1128.
- Hetherington S., McGurik A., Powell G., Cutrell A, Naderer O., Spreen B. Hypersensitivity reactions during therapy with nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001 Oct. 23(10), 1603-14.
- Hughes, S., Hughes, A., Brothers, C., Spreen, W., & Thorborn, D. (2008). PREDICT-1 (CNA106030): the first powered, prospective trial of pharmacogenetic screening to reduce drug adverse events. Pharmaceutical Statistics, 7(2), 121–129. https://doi.org/10.1002/pst.286.
- Mallal, S., Phillips, E., Carosi, G., Molina, J.-M., Workman, C., Tomažič, J., … Benbow, A. (2008). HLA-B*5701 Screening for Hypersensitivity to Abacavir. New England Journal of Medicine, 358(6), 568–579. https://doi.org/10.1056/NEJMoa0706135.
- Marfatia, D. Y., & Makrandi Smita, D. (2005). Adverse drug reactions (adr) due to anti-retrovirals (arv) : issues and challenges. Indian J Sex Transm Dis, 40(1), 5. Retrieved from medind.nic.in/ibo/t05/i1/ibot05i1p2.pdf.
- Zhang Y., Mei H, Wang Q, Xie J, Lv J, Pan X. Peptide binding Specificities of HLAB*5701 and B*5801 Sci China Life Sci, 55(9): 818-25.
Discuss